The periplasmic nitrate reductase catalytic subunit (NapA) belongs to the DMSO reductase (DMSOR) family of the molybdenum cofactor (Moco) containing enzymes and catalyzes nitrate reduction to nitrite. Some members of the DMSOR family (e.g., DMSO reductase, formate dehydrogenase, nitrite oxidoreductase, and arsenate reductase) catalyze the reversible interconversion of the substrate and product with varying efficiency. Here, we demonstrate that NapA not only can reduce nitrate to nitrite but also can also oxidize nitrite to nitrate. At least three mechanisms involving the oxygen atom transfer (OAT) process have been proposed for NapA-catalyzed nitrate reduction. NapA can also oxidize nitrite via an OAT process. However, there is no experimental evidence for any of these OAT processes using isolated pure enzymes so far. Here, we provide the first experimental evidence for OAT reactions in both forward (i.e., nitrate reduction) and backward (i.e., nitrite oxidation) reactions catalyzed by NapA using isotope labeling, mass spectrometry, and NMR experiments. Finally, we propose a mechanistic scheme supported by experiment and computation consistent with atom transfer as the rate-limiting step.
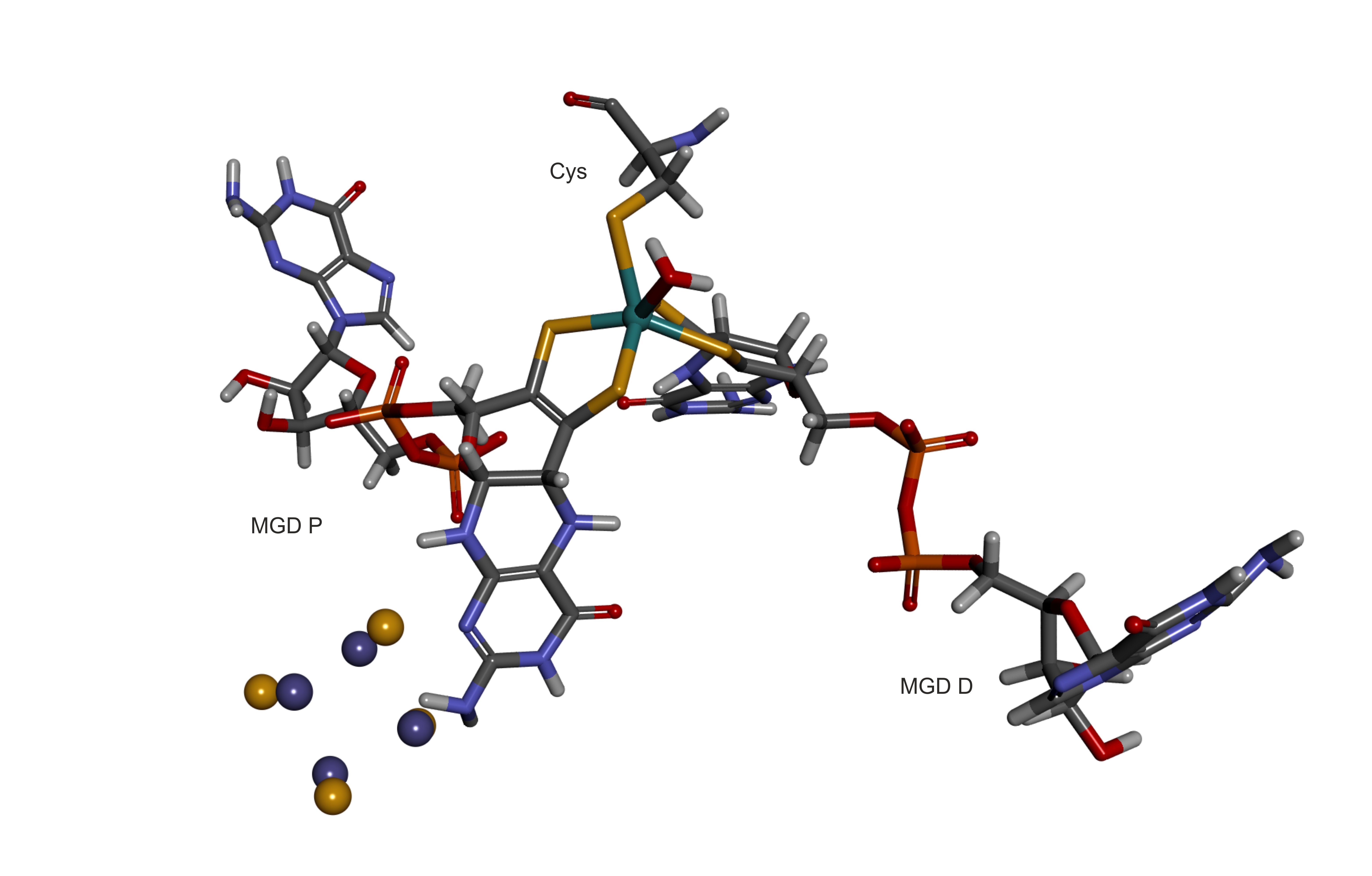
Thus, the MGD cofactor is a complex of Mo(H2O)(MGP)(MGD) and protein residue Cys (CYM):

The accompanying Fe4S4(Cys)4 cluster is [Fe4S4]2+(Cys)4 cluster was taken from Carvahlo et al. J. Chem. Inf. Model. 2014, 54, 2, 613620 https://doi.org/10.1021/ci400718m [doi.org] with corrections provided in 10.1021/acs.jcim.5b00390 [pubs.acs.org].
The missing nonbonded parameters were taken from Rappe, A.K., et al. JACS 1992, 114, 10024
The parameters work provided the following scheme is used:
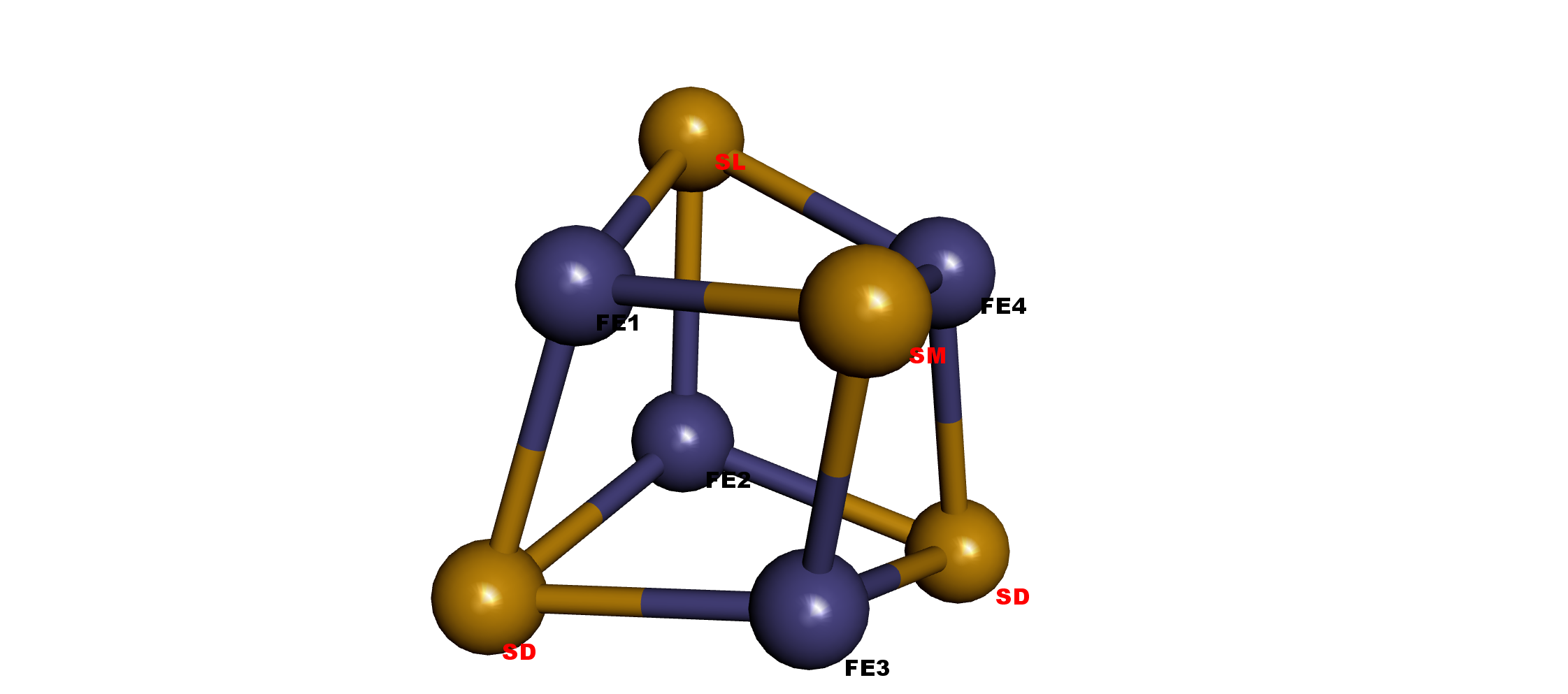
Names
Types
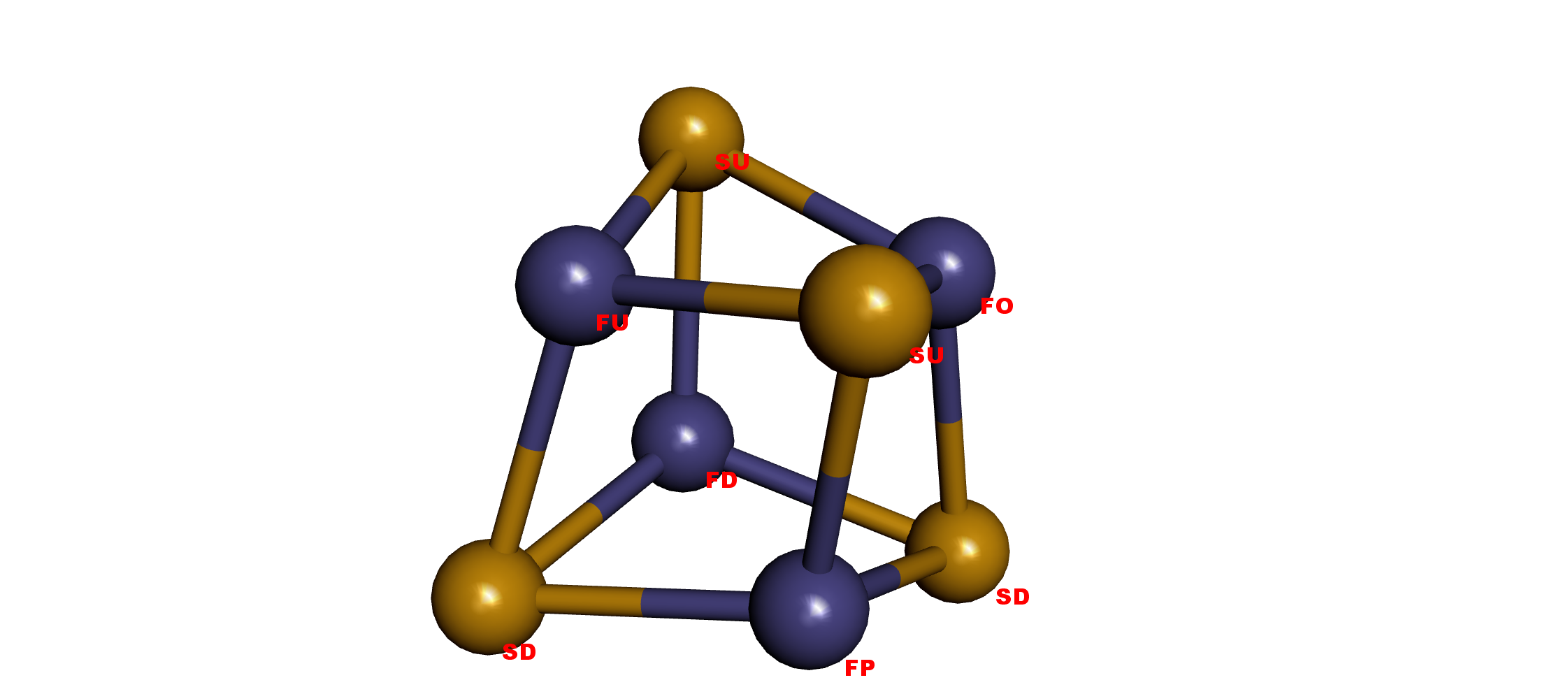
The corresponding cysteines CyA-D connect with Fe1-4, respectively.
Here is a final PDB so one can get an orientation on how the various parts link together.